What is the ozone layer?
The ozone layer is one of the outer layers embedded in the Earth’s atmosphere and is composed of a naturally occurring molecule called the ozone. The ozone, consisting of three oxygen atoms, is a molecule that makes up the gaseous layer in the Earth’s upper atmosphere, known as the stratosphere.
The purpose of the ozone layer is to protect us against the dangeroussun’s harmful rays. The ozone layer protects us from harmful radiation reaching the Earth as it diverts the radiation back into space. The goal of the ozone layer is to cover all living from harmful UV or ultraviolet rays that emerge from the sun. If there was no ozone, millions of people would suffer from debilitating skin illnesses such as skin cancer and have poor immune systems.
What is ozone depletion?
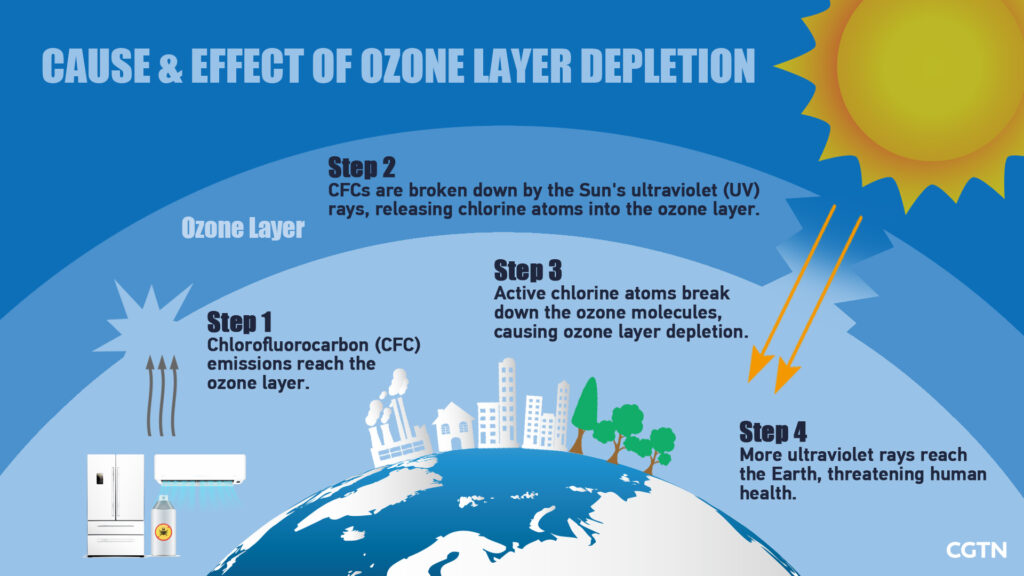
Ozone depletion refers to the lack of the Earth’s ozone layer, which means the gradual thinning and loss of the ozone layer in the stratosphere, which is the Earth’s upper atmosphere. The depletion is caused by excessive production and release of harmful chemical compounds that contain chlorine or bromine in the gaseous form and are released from various industries and other substances.
The depletion of the ozone layer is most prominent in polar regions, particularly Antarctica. It is a cause of serious concern because a deficit in the ozone layer raises the amount of ultraviolet radiation that ultimately reaches Earth and affects all living things.
When it became a cause of serious concern, the Montreal Protocol was the first thorough argument made at an international level that agreed to stop the production of chemicals that contributed to the depletion of the ozone layer. In the beginning, these chemicals included chlorofluorocarbons, and then later, they came to include methylbromide, halons, carbon tetrachloride (CTC) and hydrochlorofluorocarbons or HCFCs.
A total of 197 nations took part in the Montreal Protocol, which has worked effectively in the sense that it has reduced about 97% of the global consumption and production of controlled ozone-depleting substances or ODS.
What are the effects of the ozone layer deficit?
The immediate result of ozone layer depletion is the increased reach of UVB radiation that penetrates the ozone layer and reaches the Earth. It can have several effects on the inhabitants of the Earth. The most important one is the effect of UV radiation on human health. If the ozone layer is not protected, the impact of ozone layer depletion could be lethal.
● One prominent effect of UV rays on the human body is skin cancer, which is strongly associated with UV-B exposure. Scientists estimate that a 1% decrease in stratospheric ozone would increase the prevalence of skin cancers by 2%.
●In addition, it also increases the chances of ocular cortical cataracts.
●The ozone layer depletion also has significant effects on plants, including adverse effects on the developmental and physiological processes.
● Ozone Layer depletion can also have significant effects on marine ecosystems. For example, phytoplankton makes up the base of the aquatic food webs. However, it has been shown that exposure to UVB radiation can affect the orientation and motility of phytoplankton, resulting in decreased survival rates for many organisms.
●Exposure to UVB radiation has been shown to severely impact the development of shrimp, crabs, fish, amphibians and various other marine animals.
●UVB radiation also has a significant impact on biogeochemical cycles. It affects terrestrial and aquatic biogeochemical cycles by changing the sources and the sinks of greenhouse gasses and other necessary gasses such as carbon dioxide, carbon monoxide, ozone and carbonyl sulfide. It could result in a concentration of these gasses in the atmosphere.
●UVB radiation also significantly impacts synthetic polymers or naturally occurring bio-polymers as well as other materials of commercial interest. The radiation can increase or accelerate the breakdown of these materials, thus limiting the duration for which they’re safe to use outdoors.
What are the solutions to protect the ozone layer?
Since the depletion of the ozone layer causes the entire planet to be vulnerable to the following consequences, we must do our best to protect and help the ozone layer heal. Some of the solutions to this problem are listed below.
● Reduce the use of vehicles. As we know, all vehicles discharge vast amounts of greenhouse gasses, which can eventually condense into smog and then contribute to the depletion of the ozone layer. Therefore it is crucial to restrict the number of vehicles on the road.
● Prohibit the use of nitrous oxide. While the Montreal Protocol of 1989 significantly limited the use of chlorofluorocarbons, it did not mention nitrous oxide. This hazardous gas contributes significantly to the depletion of the ozone layer.
●Make use of eco-friendly cleaning products. Most cleaning products on the market are composed of harsh chemicals that eventually end up in the atmosphere and contribute to the ozone layer’s depletion. Therefore it is essential to use ecologically friendly cleaning products.
● Limit the use of pesticides. Although pesticides can be effective for the farming community in controlling pests and weeds, they also contribute significantly to the ozone layer’s depletion. The alternative is to use natural solutions or eco-friendly pesticides.
● Avoid buying ozone-depleting products. Several products in the market contain aerosol that is composed of chlorofluorocarbons. For instance, your fire extinguisher could have halon or halogenated hydrocarbon.
●Get rid of appliances such as air conditioners and freezes that contain chlorofluorocarbons.
●Switch to renewable sources of energy. Renewable energy is an essential step in fighting the depletion of the ozone layer.
●Several fossil fuels, including coal, which constitutes a considerable portion of the electricity production, release a significant amount of chlorofluorocarbons, depleting the ozone layer.
●Develop the practice of reusing and recycling. We aim to reduce purchasing new items and try repurposing as many of our materials as possible. If we cannot use the product, it should be recycled for another use. It will help us reduce the demand for natural resources and thus prevent the ecosystem’s exploitation and the adverse effects.
Recovery of the ozone layer
Global recognition of the harmful effects of the depletion of the ozone layer caused by chemicals such as hydrofluorocarbons, chlorine, and bromine led to an international effort to decrease the production of CFCs and other hydrocarbons. The 1987 Montreal Protocol on Substances that Weaken the Ozone Layer was particularly effective in phasing out the use of CFCs.
The Montreal Protocol was later amended several times to strengthen the decrease of CFCs and hydrocarbons further. This was effective, and by 2005, the production and consumption of ozone depleting chemicals were reduced by 90 to 95% in the countries that signed the protocol.
In 2018, the United Nations published a report that estimated that the Antarctic ozone hole would eventually close and the stratospheric ozone concentrations would return to the values in 1980 by the 2060s. Likewise, the ozone levels over the Arctic are supposed to return to 1980 by the mid-2030s.
However, a more thorough change and ozone levels would also depend on other significant changes in the atmosphere’s composition, such as carbon dioxide, methane and nitrous oxide levels. In 2014, scientists observed a tiny increase in stratospheric ozone levels for the first time in 20 years. In 2016, it was published that ozone concentrations in the stratosphere have been increasing since 2000, and the Antarctic ozone hole has been decreasing.
Conclusion
The ozone layer is between 15 to 30 kilometres above the Earth’s surface, and its function is to protect living things from the harmful effects of UV radiation. Given the consequences of ozone layer depletion, the Montreal Protocol effectively curbing the production and use of toxic chemicals and contributing to the ozone layer’s recovery. However, we do have a significant way to go, and there are several steps that we can take to protect the ozone layer ourselves.
FAQs
1. What is the ozone hole?
The ozone hole is a hole over certain parts of the Earth, including the Antarctic and the Arctic Ocean. The ozone hole allows a significant amount of ultraviolet radiation, or UVB radiation, to enter the Earth’s surface and cause a significant negative impact on living things. The ozone hole has been known to be significantly huge in the spring season, therefore, causing excessively long summers.
2. When is World Ozone Day?
The United Nations General Assembly decided to designate September 16 as the International Day for conserving the ozone layer or World Ozone Day in 1994. This day is significant as the Montreal Protocol signed on this day in 1987.
3. What are Ozone Depleting substances?
Ozone-depleting substances directly lead to the depletion of the ozone layer and the ozone hole. They include manufactured chemicals such as halocarbons, refrigerants, propellants, solvents, and foam-blowing agents such as halogens at HCFCs and CFCs.